On March 7, 2022, the government announced one of the biggest settlements ever involving the Medicaid Drug Rebate Program: Mallinckrodt, which just emerged from bankruptcy, has agreed to pay $233.7 million over seven years to resolve allegations that it schemed to avoid paying inflationary rebates to the Medicaid program for its drug, Acthar Gel.
Mallinckrodt and its predecessor, Questcor, raised the price of Acthar Gel from approximately $50 per vial in 2001 to $40,000 per vial today. Here’s a graph of the price history since 1990, when the Medicaid Drug Rebate Program started:
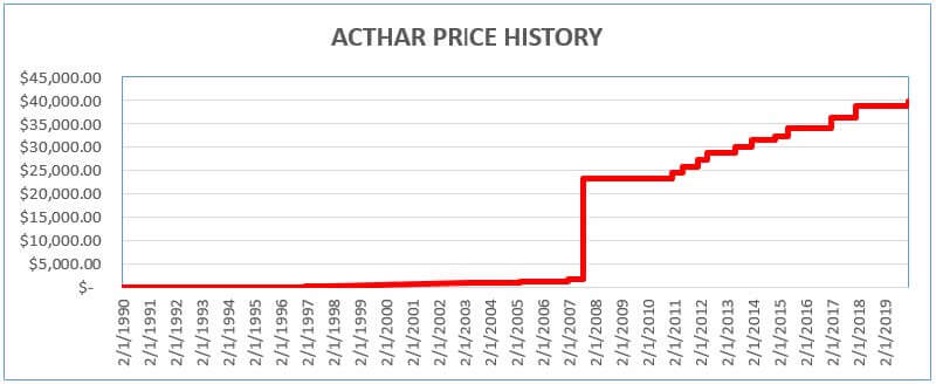
Under the rules of the Medicaid Drug Rebate Program, Medicaid was required to cover this drug, but Mallinckrodt was required to rebate back to Medicaid the difference between what Medicaid paid and what Medicaid would have paid if the price of the drug had gone up only at the general rate of inflation. See 42 U.S.C. § 1396r-8(c)(2)(A). Given the steep price increases Mallinckrodt and Questcor orchestrated, the rebates back to Medicaid should have nearly matched Mallinckrodt’s revenues from sales of the drug to Medicaid beneficiaries – if Mallinckrodt had played by the rules.
But Mallinckrodt did not play by the rules. Instead, it played an all-too-common pharmaceutical industry game called “resetting the base AMP,” where, after a huge price increase, the pharmaceutical company claims that the drug has suddenly transformed into a new drug with a new base Average Manufacturer Price. With a new base AMP, Medicaid ends up paying for the drug at its inflated price and the pharmaceutical company’s enormous inflationary rebate obligations suddenly disappear. It’s a particularly cynical way that some pharmaceutical companies rip off the government’s health care program for the poor.
In the case of Acthar Gel, as the government alleged in its complaint against Mallinckrodt, “[r]ather than paying rebates based on the extraordinary increases in Acthar’s price since 1990, Mallinckrodt began in 2013 to pay rebates on Acthar as if it had first marketed the drug in 2013.” Of course, by 2013, Acthar already had been on the market for decades. Mallinckrodt contended that the drug suddenly became new again in 2013 because the FDA had approved a new indication for it in 2010, but this contention had no basis in law. The FDA approves new indications for drugs all the time, but those new approvals do not make the drug new or entitle the manufacture to set a new base AMP. Mallinckrodt employees recognized as much in their internal communications about Acthar Gel.
Notably, in the settlement agreement, Mallinckrodt admitted that it had engaged in a charade. Specifically, Mallinckrodt stated that it “admits and agrees that there is only one Acthar, that FDA approved Acthar pursuant to a New Drug Application in 1952, and that Acthar was first produced, distributed, and marketed prior to 1990.” In other words, Mallinckrodt admitted that it had engaged in a massive fraud on the Medicaid program.
In 2018, a courageous former Mallinckrodt employee blew the whistle on Mallinckrodt and filed a False Claims Act qui tam suit against the company. As a reward, he now stands to receive over $40 million, if Mallinckrodt ever pays the full amount of the money it has agreed to pay.